Solved consider the following mechanism for the reaction Solved in t-butyl chloride synthesis experiment, a student Chloride butyl tert koh aqueous mechanism treated
Solved 12. Tertiary butyl chloride reacts with water at two | Chegg.com
Lab report on hydrolysis of tert-butyl chloride in polar solvents
Solved: calculate the percent yield of t-butyl chloride mass = 0.505g
Solved a. 1. sketch the reaction coordinate diagram for theSolved 2. t-butyl chloride reacts with water according to 9.4: the effect of temperature on reaction rateReaction activated collision libretexts kinetics potential.
Chemistry archiveThe hydrolysis of t-butyl chloride in an organic solv… Solved experiment 7: preparation of t-butyl chloride theWrite the mechanism of the reaction when t.
Solved: write the sn1 reaction for tert-butyl bromide + naoh, tert
Half life of t butyl chlorideSolved consider the substitution of t-butyl chloride Solved d) consider the reaction of tertbutyl chloride withCloruro terc-butílico, +98 %, thermo scientific chemicals.
Solved consider the following mechanism for the reactionSolved consider the following mechanism for the reaction Solved summarized procedure this can be in the form of aSolved 5) the diagram below represents the energy diagram.
Solved 1) if the t-butyl chloride is not dried before
What is the effect of each of the following on theSolved consider the following mechanism for the reaction Butyl chloride ethanol reacts chloro sn1 mechanism chemistry solventChloride butyl tert synthesis carbocation chemistry.
Chemistry of living things jeopardy template13+ to the energy diagram." references Solved in the t-butyl chloride experiment, if a student usesSolved: text: 07 question (3 points) below is the reaction between t.
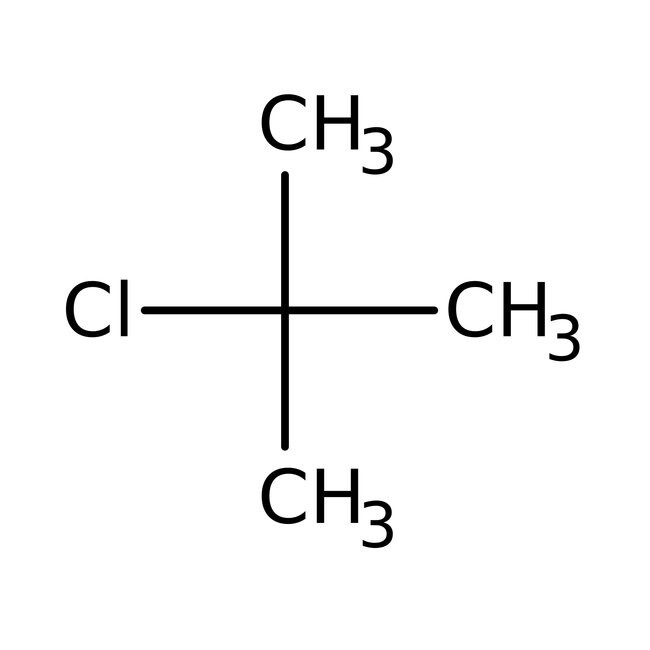
Solved the hydrolysis of tert-butyl chloride is given in the
Hydrolysis of t-butyl chlorideHydrolysis of t-butyl chloride lab report.docx One part of chemistry: synthesis of tert-butyl chlorideButyl tert hydrolysis chloride lab report mechanism transition state hydrogen ion polar solvents discussion.
.







